Global Leaders in Iron Quantification
Over 15 years with pharmaceutical companies, hospitals, research institutions, clinicians, radiologists, and researchers in disease areas such as thalassemia, sickle cell disease, MDS, cancer survivors, hereditary hemochromatosis, and other conditions.
How FERRISCAN Can Help You

For Clinicians
Discover why FerriScan is internationally recongised as the gold standard in liver iron concentration (LIC) measurement.

For Patients
Want to know more about FerriScan? Search through our frequently asked questions and learn more today.
For Pharma
Resonance Health has been providing expertise in imaging core lab services and CRO services to pharmaceutical companies for their clinical trials for over 15 years. The company has provided and is providing services for many multi-national, multicenter studies. Please contact us to discuss your needs and how we can help.
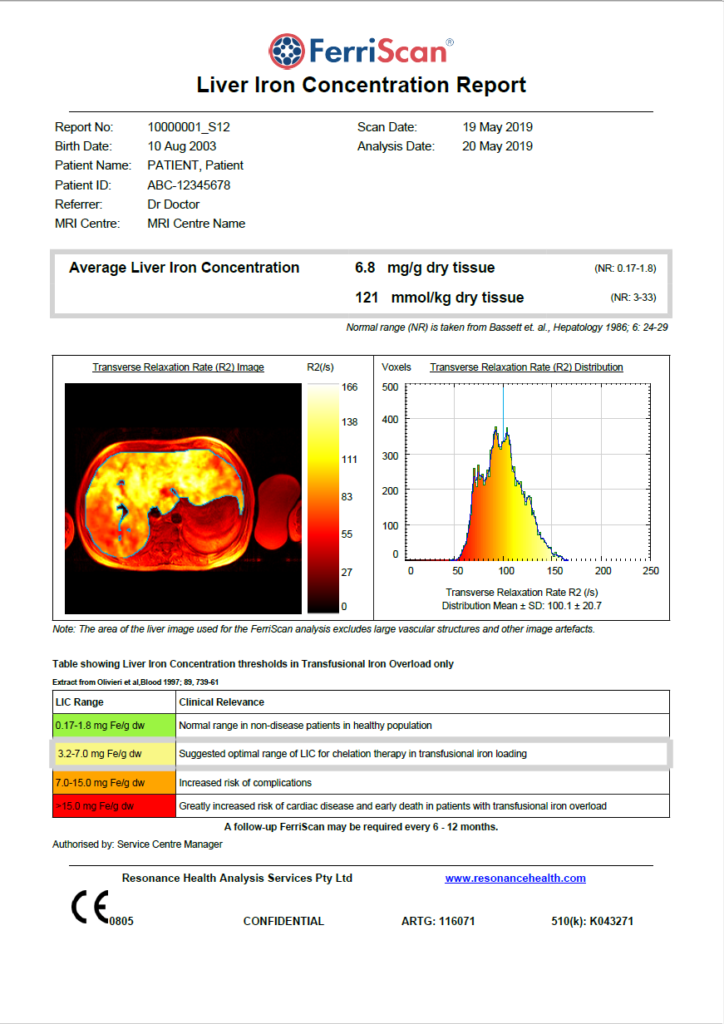
The Gold Standard in Liver Iron Concentration
FerriScan is internationally recognised as the gold standard in liver iron concentration (LIC) measurement and is included in a number of international Standards of Care.
FerriScan is a system for quantifying liver iron concentration (LIC) from specially acquired MRI images. The images used are standardised R2-MRI images (acquired using the ‘FerriScan protocol’) and proprietary internationally regulatory-cleared software is used to quantify liver iron concentration (LIC). Analysis is performed under stringent, quality controlled conditions (ISO 13485:2016 certified) in a central core laboratory by a team of highly trained engineers and physicists. FerriScan analysis has the highest sensitivity and specificity over the range of liver iron concentrations of any MRI-based method of LIC measurement, producing results that are reliable and reproducible over time and across different makes and models of MRI scanner. To date, FerriScan has been used to non-invasively measure LICs in over 50,000 patients.
FerriScan has international regulatory clearances. It was granted clearance by the FDA (US) for the quantification of LIC in 2005. In January 2013 FerriScan gained an additional clearance from the FDA as a companion diagnostic to aid in the identification and monitoring of non-transfusion-dependent thalassemia patients receiving therapy with deferasirox. FerriScan also has clearances form the TGA (Australia) and CE Mark (Europe).
The FerriScan Report
FerriScan was originally calibrated against liver biopsy in patients with various iron overload conditions.
- The FerriScan technique is robust
- There is no shift in accuracy or precision across different MRI scanners
- FerriScan is unaffected by fibrosis or chelation therapy
- FerriScan can be used for patients of all ages including neonates
An increasing number of patient treatment guidelines for thalassaemia, sickle cell disease, myelodysplastic syndrome and haemochromatosis recommend using FerriScan to quantitatively measure liver iron concentration.
About Us: Resonance Health